EfficientSeparation of cis-and trans-1,2-DichloroetheneIsomers by AdaptiveBiphen[3]areneCrystals
Yiliang Wang+,a Kaidi Xu+,a,bBin Li,a,b Lei Cui,a Jian Li,a Xueshun Jia,aHongbin Zhao,a Jianhui Fang,a and Chunju Li(李春举)*a,b
aSchoolof Materials Science and Engineering, Center for Supra-molecular Chemistry andCatalysis and Department of Chemistry Shanghai University, Shanghai 200444 (P. R.China)
bCollegeof Chemistry, Key Laboratory of Inorganic-Organic Hybrid Functional Material Chemistry,Ministry of Education, Tianjin Key Laboratory of Structure and Performance forFunctional Molecules, Tianjin Normal University, Tianjin 300387, People’s Republicof China
Angew.Chem. Int. Ed.2019, 58, 10281 –10284.
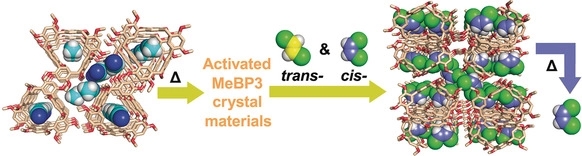
Abstract: Reported here is the highly efficientseparation of industrially important cis‐and trans‐1,2‐dichloroethene (cis‐DCE and trans‐DCE)isomers by activated crystalline 2,2′,4,4′‐tetramethoxylbiphen[3]arene (MeBP3) materials, MeBP3α. MeBP3 can be synthesized in excellentyield (99 %), and a cyclicpentamer is also obtained when using 1,2‐dichloroethaneas the solvent. The structure of MeBP3 in the CH3CN@MeBP3 crystaldisplays a triangle‐shape topology, forming 1D channels throughwindow‐to‐window packing.Desolvated crystalline MeBP3 materials, MeBP3α, preferentially adsorb cis‐DCE vapors over its trans isomer. MeBP3α isable to separate cis‐DCE from a 50:50(v/v) cis/trans‐isomer mixture,yielding cis‐DCE with a purity of96.4 % in a singleadsorption cycle. Single‐crystal structuresand powder X‐ray diffraction patterns indicate that theuptake of cis‐DCE triggers a solid‐state structural transformation of MeBP3, suggesting the adaptivityof MeBP3α materials during the sorption process. Moreover, the separation canbe performed over multiple cycles without loss of separation selectivity andcapacity.
链接:https://doi.org/10.1002/anie.201905563
 目的地搜索
目的地搜索